What You Should Know About CSV in Pharma
Por um escritor misterioso
Descrição
Learn more about computer system validation, which is required by the FDA and other global regulatory bodies for drug and medical device manufacturers.

Understanding FDA's CSA Guidance in the Context of Current Regulations and GAMP® American Pharmaceutical Review - The Review of American Pharmaceutical Business & Technology
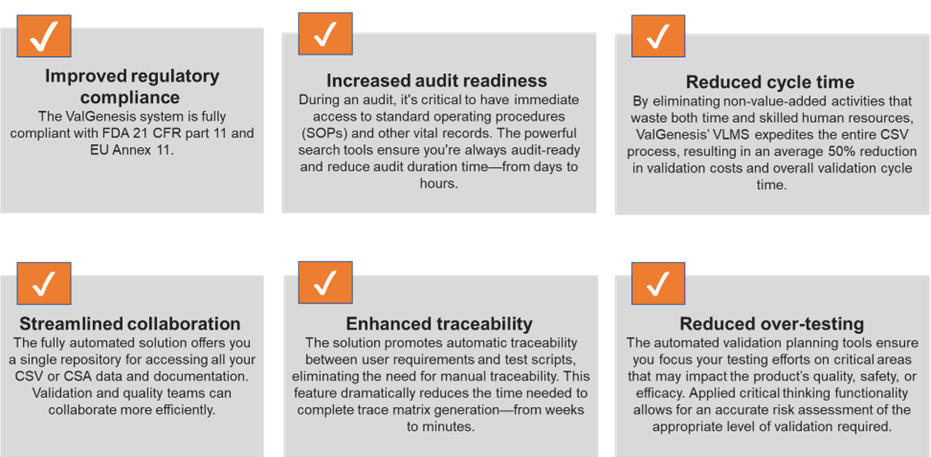
6 Common Challenges of Paper-Based computer system validation (CSV) and How to Digitize Them - Astrix
CSV is a Profession Too

What You Should Know About CSV in Pharma

From CSV to CSA - what should you know about the new validation paradigm?

Computer System Validation (CSV): Your Path to Documenting Data Integrity

Importance of computer system validation (CSV) in Pharmaceutical Industry
Data Integrity in the Pharmaceutical Industry
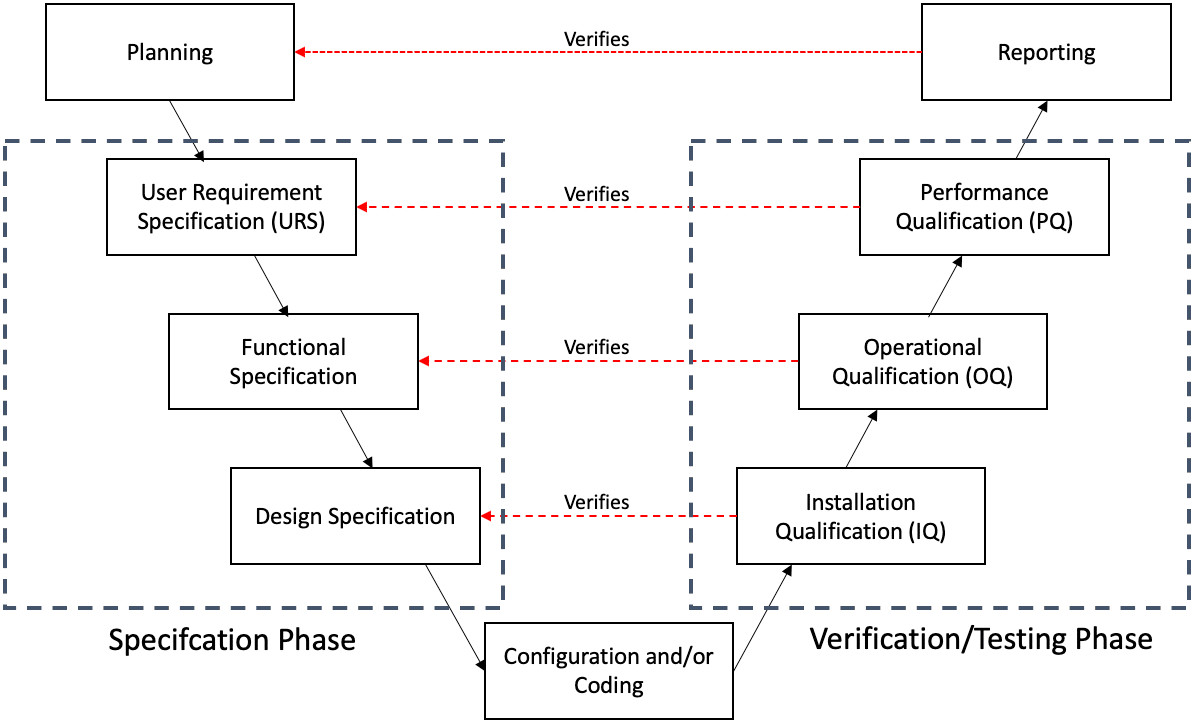
What is Computer System Validation CSV in the Pharma Industry?
Fiverr freelancer will provide Other services and do pharma, medical device, qms,validation,csv documentation, within 7 days
do pharma, medical device, qms,validation,csv documentation
Download Program, Are you compliant with FDA requirements for CSV, Data Integrity & Process Validation? Today's regulators are applying more

CSV, Data Integrity & Process Validation Virtual Summit, 2021
de
por adulto (o preço varia de acordo com o tamanho do grupo)